This blog discusses results from our survey exploring researchers’ experiences when accessing health data, finding less than half of respondents were completely successful in accessing the health data they requested. Respondents cited a range of barriers, but also offered valuable suggestions on changes that could be implemented to facilitate the safe and efficient access to health data for research.
The opportunity: To improve cancer research, patient care and outcomes
Every year almost 400,000 new cases of cancer are diagnosed in the UK. From the point a person first makes contact with the health system a whole range of information is collected, be it at the time of diagnosis, throughout the treatment pathway or as part of the long-term follow-up –this is the case not just for cancer, but for all diseases. This means there is health information, or health data, from millions of people that has been collected over the years and continues to be collected. Using this wealth of information to accelerate cancer research presents a massive opportunity to improve our understanding of cancer, the way we treat patients and ultimately to improve patient outcomes.
To seize this opportunity, it is important to understand what experience researchers have when they try to access health data. Different types of health data are held by different government structures or organisations, each with different routes to applying for access and with unique requirements. There is anecdotal evidence that accessing health data can be challenging and time consuming for researchers, however, to our knowledge, there has been little work to capture and understand the researcher’s perspective in order to then systematically address the barriers they face. Therefore, the National Cancer Research Institute (NCRI), a partnership of 18 of the largest cancer research funding organisations in the UK, and Cancer Research UK (CRUK), one of NCRI’s charity partners, are working together to survey the cancer research community about their past experiences of trying to access health data and their anticipated future needs. We hope that identifying the barriers that researchers face when trying to access various data types will enable effective solutions to overcome these and in turn promote the safe and efficient use of health data for cancer research.
Understanding researchers’ experiences
The responses we received provide an invaluable insight to the health data landscape and offer many opportunities for improving on the current health data access process across numerous data types.
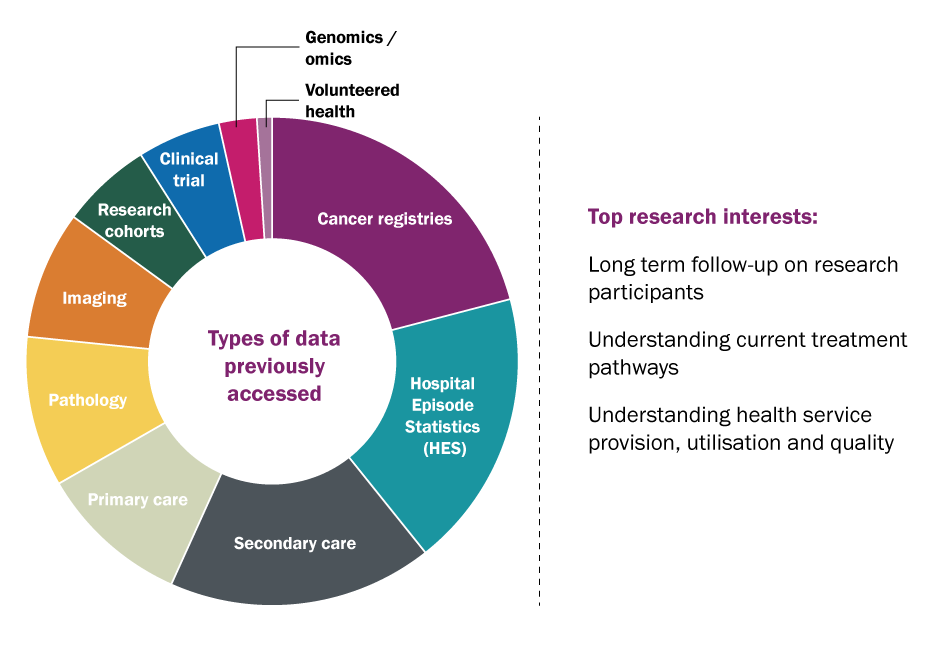
Fig.1 Left: The types of health data that researchers responding to the survey accessed or attempted to access. Right: The top three research interests that respondents gave as the reason for wanting to access the health data.
Across all data types, less than half of the researchers that responded to our survey were successful in accessing the data they were hoping to obtain (47%), and even those successful listed numerous obstacles they had to overcome in order to access the data they needed. 43% of researchers were partially successful, meaning they got access to some of the data or their data requirements were restricted in some way during the process, and 10% were completely unsuccessful in getting access to health data for research. The most common data types accessed for research purposes were; secondary care data, specifically Hospital Episode Statistics (HES), and data from cancer registries.

Access to these data types needs to be applied for through application forms or processes that were overall flagged as sometimes being unclear and therefore time-consuming, as highlighted by one respondent who felt that “every database owner has different requirements and accessing the data is so laborious & time-consuming that it hampers important research”, while another added; “you have to say up front exactly what (data) you want, not knowing what might be available. The whole process is too unclear and time consuming and is a disincentive to using the data”. This is in line with the highest listed barrier across all data types being ‘time and logistics for access’, closely followed by ‘data sharing agreement issues’.
Overcoming the barriers to data access
Several solutions to existing barriers were put forward by respondents to the survey, who also highlighted opportunities for data owners or custodians to provide more support throughout the health data access process. There was a strong push for “clear and efficient data access guidelines” to address the apparent lack of clarity around who owns the data, what data is accessible and how much it will cost to access the data and maintain access. Researchers and clinicians are advised to seek guidance on their projects early to ensure the quality of the proposal is sufficient and the data request is reasonable, but it is not always clear who to contact or what constitutes a reasonable request. It seems often researchers feel left ‘in the dark’ regarding health data, which is probably also why ‘support through data access process’ was the most listed response when researchers were asked where they felt more help was needed.
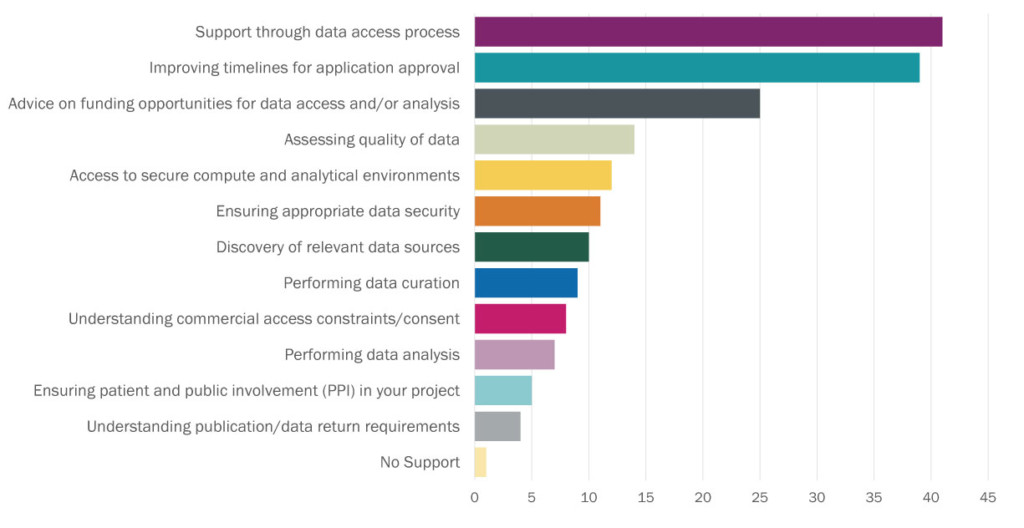
Fig.2. Researchers were asked “Considering your needs for accessing health data in future, where do you feel you would need the most support?” This graph summarises the responses given.
There were also more specific suggestions for how to make the whole process of accessing health data more efficient, such as developing “a national data passport” for researchers that demonstrated responsible use of health data for research in the past and therefore “allows them access to accelerated and proportionate data access processes”. Similarly, it was suggested specifically for clinical trial data, which often requires researchers outside the trial management team to arrange data sharing agreements with individual NHS Trusts or clinical trial units, to facilitate the “central submission of trials data to a database that can be applied to for release”. This data should then also be linked to cancer registry data to allow for the long-term follow-up of research participants, a key request from many researchers who feel this would enable them to make the most of the data that is collected through clinical trials and obtain key insights that could translate to greater patient benefit.
Improved communication and guidance, single access systems, standardised contracts and incentives for demonstrating robust data governance are all implementable suggestions that could accelerate researchers access to health data and thereby progress in cancer research. It is also worth noting that issues around the process for accessing health data are not unique to the research community, but that other sectors face these as well. This is highlighted in the Medicine Discovery Catapult’s report “Use of health data by the life sciences industry – a UK perspective” released earlier this year in collaboration with the Association of the British Pharmaceutical Industry (ABPI). Industry also regards the health data collected by the NHS as a critical asset and it is recognised that the ethical, consented and trusted use of health data by the life sciences industry could be key to developing new therapies, but they too find that miscommunication and inefficiencies during the data access process result in significant delays.
Working with researchers, data custodians and NCRI Partners
With the ambition to beat cancer sooner and accelerate research, it is vital that these application processes are reviewed so that important research can be facilitated without compromising data quality, researcher accessibility and patient confidentiality. NCRI is committed to supporting researchers, data custodians and the NCRI Partners in their endeavours to transform the ability of researchers to safely access high-quality cancer data for research in an effort to change all our lives for the better. We will conduct a thorough analysis of the survey responses and create a comprehensive report so that insights gained can be communicated to data custodians and the wider research community.
My personal view that developed while working on this topic during my internship is that the responsibility to make data accessible to researchers lies not only with those that hold and protect the data, but also with the researchers and with us, the public. Safe and efficient access to health data for research needs to be a collaborative effort where data custodians clearly communicate the requirements for a successful application and process these not as guardians of data, but as a facilitators of research, while at the same time researchers need to ensure their data requests are well founded, thought out, widely consulted on (ideally including with patients or patient advocates) and adhere to the requirements set out by the data custodians. There is nothing to be gained from pointing fingers, what really needs to happen is for the organisations that hold data and the researchers that use data to come together and work out how to make data access processes work for everyone involved. There are already good examples of this happening and real enthusiasm to make this work: NHS Digital’s Research Advisory Group and NHSX have been set up to improve health data services and address structural barriers to accessing NHS data; The Get Data Out programme run by the National Cancer Registration and Analysis Service (NCRAS) makes key cancer statistics for small groups of patients publicly available; Health Data Research UK is working hard to make data more accessible for research through its Health Data Research Alliance, Innovation Gateway and Digital Innovation Hubs.
Finally, we all need to be aware of the advancements in patient care that can be realised by us consenting to our health data being used for meaningful research. Ultimately, we will all be confronted with ill health at some point during our lives and will rely on our health system for help, either as a patient, a carer, family member or friend. When that time comes, we need the NHS to be the best it can possibly be and by consenting for the safe use of our health data for research purposes we can do our bit to get it there. In turn our health data should then be safely accessible to researchers so that they can readily use it to make important discoveries that improve our understanding of cancer and the way we treat patients.
“Having recently graduated from Loughborough University with a degree in Human Biology, I was eager to gain some experience within the charity sector. The internship in the NCRI’s Strategy & Initiatives team appealed to me greatly, with it allowing for the flexibility to explore different projects according to my interests and providing the opportunity to have an impact on different areas of cancer research. As the NCRI Strategy Intern, I was extremely interested to further understand the rise of big data in the healthcare industry as it shows great promise and potential to accelerate cancer research.”
Brooke Butler, NCRI Strategy Intern
If you would like to engage with us on this work or are interested to find out more, please get in touch: strategy@ncri.org.uk